In a Texas operating room on August 11, 2025, a newly FDA-approved gene therapy treatment was surgically implanted in the eye for the first time outside a clinical trial. The treatment is called Revakinage tarortcel-lwey (EnceltoTM) and is sold by Neurotech Pharmaceuticals. Encelto is first of its kind in ways that should be of interest to SBM readers:
- It’s a new treatment for an unmet medical need.
- It represents a new drug-delivery platform.
- It’s an expansion of the scope of gene therapies.
What is Encelto?
Quoting the Package Insert: “ENCELTO is an allogeneic encapsulated cell-based gene therapy indicated for the treatment of adults with idiopathic macular telangiectasia type 2 (MacTel).”
What do all those fancy-sounding words mean?
“…cell-based gene therapy…”
“Cell based gene therapy” refers to the fact that the therapeutic activity of this product is produced by genetically modified cells. The cells are human Retinal Pigment Epithelial (RPE) cells. The RPE is the outermost layer of the retina. It performs crucial functions necessary for survival and proper function of the photoreceptors. In contrast to the other layers of the retina, the RPE is not part of the visual pathway. It does not contain photoreceptors (rods and cones) or neurons conducting visual signals.
For Encelto, RPE cells are genetically modified to produce ciliary neurotrophic factor (CNTF). CNTF is a protein produced within many tissues in the body, especially in the nervous system. It has numerous effects, but most relevant to MacTel and other neurodegenerative diseases is its neuroprotective effects.
…allogeneic…
“Allogeneic” means that the RPE cells come from the same species, in this case humans, but not from the patient receiving the treatment. The RPE cells are harvested then genetically modified to produce CNTF.
…encapsulated cell technology…
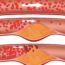
The genetically-modified allogeneic RPE cells are not implanted into the retina of MacTel patients. The cells are loaded into tiny capsules and implanted elsewhere into eyes of patients with MacTel. A semipermeable membrane in the capsule protects the cells from the patient’s immune system, allows oxygen and necessary nutrients to reach the RPE cells, and allows the CNTF to exit the capsule and migrate to the therapeutic target in the retina. The capsules are intended to be left in the eye permanently. The pivotal clinical trials studied clinical outcomes for two years, but the duration of the therapeutic effect is not yet known. CNTF levels comparable to baseline have been measured as long as 14.5 years after implantation.
technology from the product website.
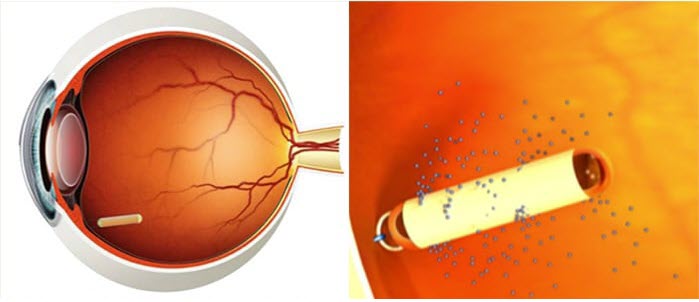
The therapeutic product, ciliary neurotrophic factor (CNTF),
diffuses into the eye via a semipermeable membrane.
https://www.neurotechpharmaceuticals.com/science-technology/
Here’s a video demonstrating the surgical implantation of Encelto. [Warning: Not for the squeamish. High resolution video of eye surgery, including blood.]
…macular telangiectasia type 2…
Macular Telangiectasia type 2 (MacTel) is the approved indication for Encelto. MacTel is a relatively rare condition. It affects both eyes and is generally first noted in middle age. Patients suffer progressive damage to the photoreceptors in the center of the retina (the macula) and central vision is gradually diminished. Telangiectasis is a description of abnormal blood vessles seen in MacTel patients, initially leading clinicians to believe MacTel was primarilty a disease of the retina blood vessels. Further research has shown that the vascular changes are secondary to metabolic defects and damage to other cells in the retina.
There is a hereditary component to MacTel, but it does not follow simple Mendelian inheritance in most cases. Mutations at multiple loci are associated with MacTel. A common pathway involves metabolism of 2 amino acids: glycine and serine.
MacTel affects the same part of the eye, and has many of the same effects as Age-Related Macular Degeneration, but is a clinically distinct entity
Expanding the scope of Gene Therapy
There are approximately two-dozen FDA approved gene therapies. Encelto is not the first gene therapy approved for a retinal disease. Voretigene neparvovec (Luxturna) was approved in 2017 for treatment of an early onset form of retinitis pigmentosa known as Leber’s congenital amaurosis.
Among the approved gene therapy drugs, there are two for sickle cell disease: Exagamglogene autotemcel (Casgevy) and Lovotibeglogene autotemcel (Lyfgenia). There are several gene therapies for treatment of cancers. There was even a gene therapy crafted for treatment of a single patient with a rare genetic disease.
Most gene therapies, to date, modify the genes within a patients’ own cells. This is to replace or repair a disease-causing gene. For instance, babies born with Leber’s congenital amaurosis lack a gene necessary for the function and survival of critical cells in the retina. The treatment for Leber’s congenital amaurosis, Luxturna, is injected beneath the retina, allowing a viral vector to introduce a functional gene into the retinal cells.
Some gene therapies for cancer — a topic worthy of a dedicated SBM post — are produced and delivered somewhat differently.
What’s so special about Encelto?
Encelto diverges from prior gene therapies in at least 2 ways. It does not involve genetic modification of the patient’s own cells, and it does not replace or repair a defective disease-causing gene. It is not belived that MacTel is a result of a genetic deficiency of CNTF. The therapeutic effect is likely due to nonspecific neuroprotective effects of CNTF.
It is instructive to think of Encelto as a sustained-release drug-delivery system. It is not just a drug reservoir, it is a factory, with genetically modified RPE cells synthesizing the drug.
The neuroprotective effect of CNTF has the potential to treat other conditions. It is being explored to treat other diseases associated with neuronal cell death in the retina and optic nerve. One could imagine Encelto, or a similar platform, being used to deliver CNTF for treatment of neurodegenerative diseases in other parts of the body. In addition, it may be possible to use encapsulated cell technology with cell modified to produce different therapeutic for other categories of diseases.
Conclusion
Encelto fills an unmet medical need for the treatment of MacTel. It also represents a diversion from existing gene therapies in ways that may prove useful to treat diseases in the eye and elsewhere.